Antihypertensive drugs could treat cerebral small vessel disease
Posted: 18 November 2021 | Anna Begley (Drug Target Review) | No comments yet
The antihypertensive drug candesartan cilexetil reduced matrisomal protein accumulation in mice with cerebral small vessel disease.
A team at the Niigata University, Japan, have revealed that the antihypertensive drug candesartan cilexetil reduced matrisomal protein accumulation and normalise vascular stiffness and cerebral blood flow in a mouse model of hereditary cerebral small vessel disease (CSVD). According to the researchers, this discovery opens up a promising new avenue for the treatment of age-related cerebral arteriopathy.
NEWS: ABI3 gene may be the key to new Alzheimer’s disease therapies
READ HERE
CARASIL (cerebral autosomal-recessive arteriopathy with subcortical infarcts and leukoencephalopathy) is a hereditary form of CSVD which is caused by dysfunction of HTRA1 (high temperature requirement A serine peptidase 1). It was initially reported as a recessive hereditary disease, but dominant traits have been reported in many recent cases. HTRA1 is also attracting attention as a risk gene for age-related CSVD.
In CARASIL arteriopathy, thickening of the intima is observed and the changes resemble age-related CSVD. The researchers therefore hypothesised that the study of the pathogenesis of CARASIL would also lead to the study of the pathogenesis of age-related CSVD.
The team found that a group of proteins called matrisomes, which consist of extracellular matrix (ECM) and ECM-binding proteins, accumulated in the cerebral arteries of aged HTRA1 gene-knockout mice. Consequently, 40 percent of the accumulated proteins were composed of matrisome proteins. The matrisome proteins were also found to accumulate in the inner membrane of CARASIL patients.
The accumulation of matrisome proteins increased significantly with age. In addition, cerebral blood vessels became stiffer, and cerebral blood flow was reduced in HTRA1-deficient mice. The accumulated matrisome proteins included fibronectin, a central component of the matrisome and a known substrate of HTRA1. “This change in CARASIL may be related to the inadequate degradation of matrisomes due to HTRA1 dysfunction and the increase in matrisomes due to ageing” proposed Professor Osamu Onodera,
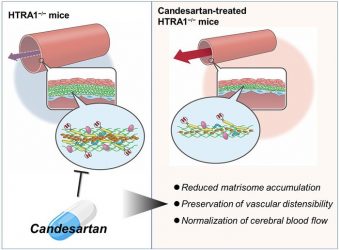
Therapeutic effect of candesartan on cerebral arteriopathy via inhibition of matrisome protein accumulation [credit: Niigata University].
The researchers examined the effects of the antihypertensive drug candesartan cilexetil, which has been reported to inhibit the accumulation of fibronectin in other organs. The results, detailed in the Journal of Clinical Investigation, showed that candesartan treatment reduced matrisomal protein accumulation and normalised vascular stiffness and cerebral blood flow regardless of its antihypertensive effect.
“What we saw was remarkable. Even when HTRA1 was completely absent, 61 percent of the accumulated matrisomal proteins were suppressed. This effect is not accompanied by an increase in degrading enzymes or suppression of matrisome protein expression. We think it is important to clarify this mechanism in the future,” explained Dr Taisuke Kato.
“Matrisomes also accumulate in other hereditary and in age-related CSVDs. A protective strategy for cerebral blood vessels to prevent matrisome accumulation with inexpensive oral drugs could be a major change for age-related brain diseases,” concluded Onodera.
Related topics
Drug Leads, Drug Repurposing, Drug Targets, Genetic Analysis, Genomics, In Vivo, Protein, Protein Expression, Small Molecules, Therapeutics
Related conditions
cerebral small vessel disease
Related organisations
Niigata University
Related people
Dr Taisuke Kato, Professor Osamu Onodera