Three-dimensional structure of the genome replication machine discovered
Posted: 3 October 2019 | Rachael Harper (Drug Target Review) | No comments yet
New research provides insights into how cancers can arise when DNA polymerase delta is not functioning properly.
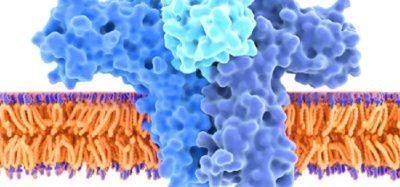
It has been discovered how the enzyme DNA polymerase delta works to duplicate the genome that cells hand down from one generation to the next. The researchers from Mount Sinai, US also found out how certain mutations can modulate the activity of this enzyme, leading to cancers and other diseases.
“DNA polymerase delta serves as the duplicating machine for the millions to billions of base pairs in human and other genomes. We were able to present for the first time a near-atomic-resolution structure of the complete enzyme in the act of DNA synthesis,” said lead investigator Aneel Aggarwal, PhD, Professor of Pharmacological Sciences at the Icahn School of Medicine at Mount Sinai. “This knowledge furthers our basic understanding of this complex enzyme which is essential for survival in higher organisms from humans to yeast.
“At the same time, our work provides insights into how cancers can arise when DNA polymerase delta is not functioning properly and offers a novel basis for designing inhibitors of the polymerase that could potentially serve as effective treatment in certain cancers.”
The researchers also mapped a number of inherited and somatic mutations in DNA polymerase delta that are associated with ‘hypermutated’ tumours. In addition to cancers, these mutations may be associated with multi-symptom mandibular hypoplasia, deafness and lipodystrophy syndrome.
…certain mutations can modulate the activity of this enzyme, leading to cancers and other diseases”
Recent advances in cryo-electron microscopy were essential to the researchers’ work. This technique allowed the researchers to examine not only individual atoms of the DNA polymerase delta but also how they move to achieve accurate replication of the genome.
Mount Sinai has said it will continue to explore the unique structure and mechanism of the polymerase, particularly its relationship to cancer and disease pathogenesis.
“We know that certain cancers become dependent on this enzyme for their survival,” said Dr Aggarwal, “and inhibiting its activity could provide a valuable therapeutic window for future medical research.”
The study was published in Nature Structural & Molecular Biology.
Related topics
DNA, Enzymes, Genomics, Therapeutics
Related conditions
Cancer, lipodystrophy syndrome, multi-symptom mandibular hypoplasia
Related organisations
Mount Sinai
Related people
Aneel Aggarwal PhD