High-throughput 3D cell migration assays in organ-on-a-chip technologies
Posted: 30 September 2021 | Désirée Goubert (MIMETAS), Dr Lenie van den Broek (MIMETAS), Luuk de Haan (MIMETAS), Thomas Olivier (MIMETAS) | No comments yet
Organ-on-a-chip models can provide an alternative to cell cultures, animal models and traditional assays. In this article, Dr Désirée Goubert, Thomas Olivier, Luuk de Haan and Dr Lenie van den Broek explore the advantages of organ-on-a-chip technologies and how they can enable the in vitro study of three-dimensional (3D) cell migration in exceptional detail.
Cell migration is an essential process for many biological phenomena such as early embryonic development and immune cell response. It also plays an important role in disease processes such as wound healing and cancer metastasis.
To accomplish the processes that are involved in cell migration, including chemoattraction, rolling adhesion, tight adhesion collective cell migration and/or extravasation and transmigration, cells must successfully navigate the complex three-dimensional (3D) environment of living tissues. This is a challenging process as cells need to move through complex or dense structures by remodelling the extra cellular matrix (ECM) and/or by undergoing specific cellular adaptations.
Classical migration models
Due to the importance of cell migration in health and disease, recent interest has focused on the detailed molecular and biophysical mechanisms that are related to 3D cell migration. To this end, many papers describe the mechanisms of this process in cultured cells on flat, two-dimensional (2D) substrates of glass and plastic. Although the outcomes of these studies can be very valuable, they do not resemble the complex physiological conditions that are present in the human body.1 Besides the lack of an ECM and other extracellular barriers in these models, they also miss the interaction with other cells and tissues and the associated signalling cascades as well as the presence of vasculature and shear flow.
The use of laboratory animals such as mice is a widely used alternative, however their biology does not always translate to humans.2 In addition, mouse models cost a lot of money and time and are ethically debatable.3
To study cell migration, 3D in vitro systems such as Transwell assays have been developed. Despite these systems incorporating more aspects of 3D cell migration than their 2D counterparts, the fact that gravity pulls the cells in the same direction as the active migration limits their usability – especially for longer experiments. Furthermore, imaging and subsequent analysis can be tedious and suffers from low throughput.4 Therefore, there is an urgent need for models that are more physiologically relevant and that allow longer lasting, real time and high-throughput experiments.
Modelling 3D cell migration using organ-on-a-chip
Progress in the development of suitable 3D cell migration models has been made in the form of organ-on-a-chip models. These models replicate key aspects of human biology that are crucial for physiological processes and drug effects. By recreating the functioning of organs, implementing tissue barrier properties and the possibility to combine multiple organ interactions, organ-on-a-chip models allow for the in vitro study of 3D cell migration in unprecedented detail.5,6
The general low number of chips means that most organ-on-a-chip migration devices are low-throughput, limiting scalability, for example, for drug screening.7
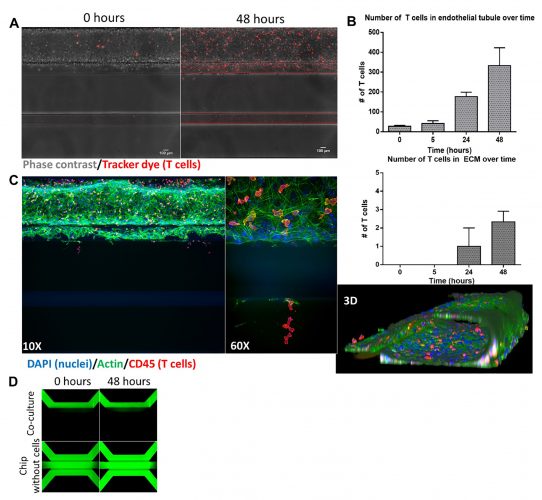
Figure 1: Co-culture of endothelial and T cells
A. Endothelial tubule (phase contrast) is perfused with T cells stained with a tracker dye that attaches to and migrates across the vessel wall during 48 hours of co-culture. B. Quantification of the number of T cells residing in the tubule (top) as well as those that migrated into the ECM (bottom) over time. C. Immunofluorescent staining of co-culture after 48 hours, imaged at 10X (left) and 60X (middle, red highlighted region) and a 3D reconstruction (right). D. Perfusion of tubule with media containing a fluorescent dextran molecule shows retainment of barrier function during 48 hours of co-culture.
Applications of 3D cell migration in organ-on-a-chip
Multiple complex models for a range of different organs and applications have been described using organ-on-a-chip technologies, including vasculature,8 angiogenesis,9 barrier integrity assays,10 3D neurite outgrowth11 and many others.12-14 Besides the increased physiological relevance, a major advantage of studying 3D cell migration in organ-on-a-chip models is the extent of control over the throughput and content of the readouts. More specifically, for screening a large number of compounds, one can choose a high-throughput approach in which several configurations can be tested in a matter of hours, while a high-content approach can be chosen to focus on the motility of individual cells as they respond to their local environment. To this end, studying 3D cell migration in organ-on-a-chip allows for a wide variety of potential read-outs and aspects to study.
One of the migration assays that can be performed using these models is the real-time tracking of fluorescently labelled cells under perfused conditions using a specialised rocker. High quality organ-on-a-chip technologies can offer excellent and easy observation and imaging of 3D cell migration, especially when utilising clear bottoms made from optical quality glass and polymers.
We were able to coculture endothelial cells with primary T cells and demonstrated the effects of a chemotactic gradient as well as inflammation on the migratory behaviour of the T cells in an organ-on-a-chip method.15 The presence of a vascular structure meant we were able to study directional T-cell migration from the vessel into the underlying tissue. We showed that T cells adhere to and migrate across the endothelial vessel wall under healthy conditions towards a chemotactic cue and that this healthy state can be changed into a disease-like state by addition of inflammatory mediators or additional cell types, resulting in massive infiltration of T cells.
The positional distribution of cells can be compared between different conditions to quantify the T-cell migration between different conditions (Figure 1B, Figure 2A).
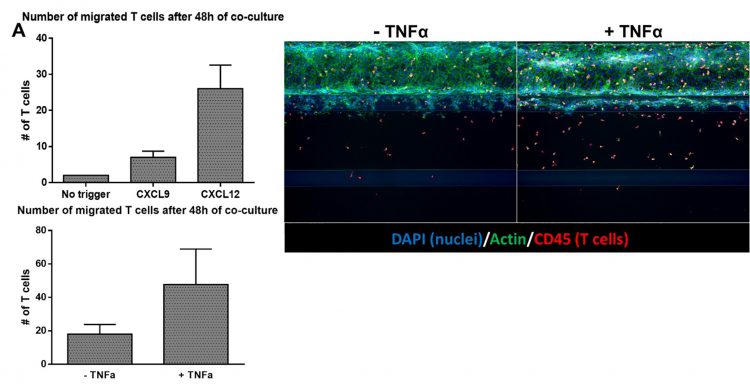
Figure 2: Migratory behaviour of T cells can be affected by addition of chemotactic cues and cytokines
A. The number of migrated T cells increases in response to chemotactic cues (top panel) and an inflammatory-like state of the endothelial vessel (bottom panel). B. Immunofluorescent staining of co‑cultures after 48 hours showing the effect of pre-incubating the endothelial vessel with TNFα.
Imaging of 3D migration in organ-on-a-chip
High-content imaging allows for detailed visualisation of biological structures, 3D reconstitution and complex analysis of 3D cell migration. Therefore, it becomes feasible and affordable to obtain multiple quantitative descriptors that could be used for comparative research into disease phenotypes and
compound effects. For proper visualisation, cells can either be fixed with formaldehyde and stained with antibodies or cells can be visualised in real time by staining them with a fluorescent cell tracker.
About the authors
 Dr Désirée Goubert obtained her bachelor and master’s in Biomedical Sciences in Hasselt University, Belgium, after which she did her PhD in Biomolecular Sciences in Groningen, the Netherlands. She has always had a passion for science communication and is responsible for content production at MIMETAS.
Dr Désirée Goubert obtained her bachelor and master’s in Biomedical Sciences in Hasselt University, Belgium, after which she did her PhD in Biomolecular Sciences in Groningen, the Netherlands. She has always had a passion for science communication and is responsible for content production at MIMETAS.
 Thomas Olivier, BSc, is a Bioinformatics Engineer at MIMETAS. Thomas has been developing and automating various imaging-based assays for use with organ-on-a-chip technologies and his assays have been used for a variety of different scientific articles, providing new insights into a variety of organ-on-a-chip models.
Thomas Olivier, BSc, is a Bioinformatics Engineer at MIMETAS. Thomas has been developing and automating various imaging-based assays for use with organ-on-a-chip technologies and his assays have been used for a variety of different scientific articles, providing new insights into a variety of organ-on-a-chip models.
 Luuk de Haan obtained his bachelor’s in Biology and master’s in Molecular and Cellular Life Sciences at Utrecht University, the Netherlands, during which he developed an interest in novel cell culture systems to study and understand human biology. As Scientist in the Model Development team of MIMETAS, he is involved in the optimisation and characterisation of various organ-on-a-chip models for a wide range of applications.
Luuk de Haan obtained his bachelor’s in Biology and master’s in Molecular and Cellular Life Sciences at Utrecht University, the Netherlands, during which he developed an interest in novel cell culture systems to study and understand human biology. As Scientist in the Model Development team of MIMETAS, he is involved in the optimisation and characterisation of various organ-on-a-chip models for a wide range of applications.
 Dr Lenie van den Broek obtained her master’s in Life Science and Technology at the Leiden University, after which she obtained her PhD at the faculty of Medicine at VU University of Amsterdam. During two postdoc positions, she further extended her knowledge in tissue engineering and 3D in vitro modelling. As Senior Scientist in the Model Development team of MIMETAS, she supervises and manages (junior) scientists who develop various 3D organ-on-a-chip assays and models to mimic human diseases.
Dr Lenie van den Broek obtained her master’s in Life Science and Technology at the Leiden University, after which she obtained her PhD at the faculty of Medicine at VU University of Amsterdam. During two postdoc positions, she further extended her knowledge in tissue engineering and 3D in vitro modelling. As Senior Scientist in the Model Development team of MIMETAS, she supervises and manages (junior) scientists who develop various 3D organ-on-a-chip assays and models to mimic human diseases.
References
- Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424(6951):870-2.
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731-8.
- Levy N. The use of animal as models: ethical considerations. Int J Stroke. 2012;7(5):440-2.
- Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014(88).
- Zhang B, Korolj A, Lai BFL, Radisic M. Advances in organ-on-a-chip engineering. Nat Rev Mater. 2018;3(8):257-278. doi:10.1038/s41578-018-0034-7
- Mondadori C, Crippa M, Moretti M, et al. Advanced Microfluidic Models of Cancer and Immune Cell Extravasation: A Systematic Review of the Literature. Front Bioeng Biotechnol. 2020;8:907.
- Pavesi A, Tan AT, Koh S, et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight. 2017;2(12).
- van Duinen V, van den Heuvel A, Trietsch SJ, et al. 96 perfusable blood vessels to study vascular permeability in vitro. Sci Rep. 2017;7(1):18071.
- van Duinen V, Stam W, Mulder E, et al. Robust and Scalable Angiogenesis Assay of Perfused 3D Human iPSC-Derived Endothelium for Anti-Angiogenic Drug Screening. Int J Mol Sci. 2020;21(13).
- Nicolas A, Schavemaker F, Kosim K, et al. High throughput transepithelial electrical resistance (TEER) measurements on perfused membrane-free epithelia. Lab Chip. 2021;21(9):1676-85.
- Spijkers XM, Pasteuning-Vuhman S, Dorleijn JC, et al. A directional 3D neurite outgrowth model for studying motor axon biology and disease. Sci Rep. 2021;11(1):2080.
- Poussin C, Kramer B, Lanz HL, et al. 3D human microvessel-on-a-chip model for studying monocyte-to-endothelium adhesion under flow – application in systems toxicology. ALTEX. 2020;37(1):47-63.
- Gjorevski N, Avignon B, Gérard R, et al. Neutrophilic infiltration in organ-on-a-chip model of tissue inflammation. Lab Chip. 2020;20(18):3365-74.
- Beaurivage C, Naumovska E, Chang YX, et al. Development of a Gut-On-A-Chip Model for High Throughput Disease Modeling and Drug Discovery. Int J Mol Sci. 2019;20(22).
- de Haan L, Suijker J, van Roey R, et al. A Microfluidic 3D Endothelium-on-a-Chip Model to Study Transendothelial Migration of T Cells in Health and Disease. International Journal of Molecular Sciences. 2021;22(15):8234.
Related topics
Drug Development, In Vitro, Organ-on-a-Chip, Screening, Small molecule, T cells, Technology
Related conditions
Cancer
Related organisations
Molecular Devices