Controlling cellular noise may stop cancer and bacterial relapse
Posted: 6 January 2026 | Drug Target Review | No comments yet
Scientists have developed a new mathematical ‘Noise Controller’ that can stabilise random cellular fluctuations, offering a potential breakthrough in preventing cancer recurrence and antibiotic resistance.

Scientists in South Korea have developed a new mathematical framework that could help explain why cancer and bacterial infections sometimes return after treatment and how future therapies might prevent it.
The research, led jointly by Professor Kim Jae Kyoung of KAIST and the IBS Biomedical Mathematics Group, Professor Kim Jinsu of POSTECH and Professor Cho Byung-Kwan of KAIST, tackles a long-standing problem in biology: random fluctuations inside cells, known as biological noise.
Even genetically identical cells can behave very differently because the molecular processes that govern life – such as protein production and degradation – are inherently random. These fluctuations can create rare ‘outlier’ cells that survive chemotherapy or antibiotic treatment, eventually leading to relapse or resistance.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
Why averages are not enough
For decades, biologists and bioengineers have focused on controlling the average behaviour of a population of cells. While this can stabilise overall protein levels, it often fails to control what happens at the level of individual cells.
“Standard control methods are like adjusting a shower,” the researchers explained. “You might get the water to average 40 degrees, but if that average is achieved by alternating between freezing cold and boiling hot water, you can’t take a shower. Similarly, in biology, getting the average right isn’t enough if individual cells are fluctuating wildly.”
These extreme fluctuations are not just inconvenient – they are dangerous. In medical treatments, it is often the rare cells that deviate from the norm that survive drugs and drive disease recurrence.
A controller for noise itself
To address this, the team developed a new mathematical design called the Noise Controller (NC). Unlike traditional genetic circuits that respond only to protein levels, this controller directly senses and regulates the noise – the variability itself.
The researchers identified a regime they call Noise Robust Perfect Adaptation, in which both the average protein level and its variability remain stable even when conditions change.
The system works by combining protein dimerization, in which two proteins bind together, with targeted protein degradation. This allows the cell to effectively measure how much its internal state is fluctuating and actively dampen those fluctuations.
Using this approach, the researchers identified a regime they call Noise Robust Perfect Adaptation, in which both the average protein level and its variability remain stable even when conditions change. Remarkably, the noise can be reduced to a fundamental physical limit, known as a Fano factor of 1, which is typically considered unavoidable in molecular systems.
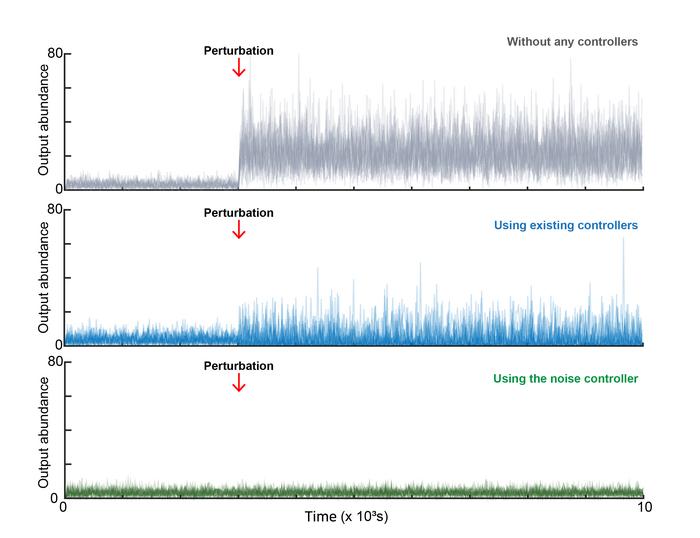

It is possible to reduce the biological noise to a desired level. Without any control mechanism, external stimuli cause the population-level average of cellular outputs to shift. Existing control strategies can maintain the population average, but the magnitude of noise at the single-cell level remains high. However, by integrating the NC, both the population average and the single-cell noise can be simultaneously stabilized and reduced. Credit: Institute for Basic Science.
Proof through simulation
The framework was tested using computer simulations of the DNA repair system in Escherichia coli. In standard conditions, around 20 percent of cells failed to activate DNA repair due to internal noise and subsequently died. When the Noise Controller was applied, the failure rate dropped to just 7 percent.
This suggests that, in principle, mathematical control could force normally unresponsive or ‘lazy’ cells to behave like the rest of the population, reducing the emergence of resistant outliers.
Implications for future therapies
“This research demonstrates that cellular noise – often dismissed as luck or unavoidable randomness – can be brought into the realm of precise mathematical control,” said Professor Kim Jae Kyoung, the corresponding author. “We expect this technology to play a key role in developing smart microbes and overcoming drug resistance in cancer therapy.”
This research demonstrates that cellular noise – often dismissed as luck or unavoidable randomness – can be brought into the realm of precise mathematical control.
Professor Kim Jinsu added, “This achievement shows the power of mathematical modelling, starting from theoretical equations to design a mechanism that solves a fundamental biological problem.”
While the work is currently theoretical, it lays important groundwork for future experimental research. By enabling control at the level of single cells, the Noise Controller could one day contribute to the development of new cancer treatments, more effective antibiotics and advanced synthetic biological systems designed to behave reliably even in noisy cellular environments.
Related topics
Cancer research, Computational techniques, Disease Research, Drug Development, Drug Discovery Processes, Microbiology, Molecular Biology, Molecular Modelling, Oncology, Synthetic Biology, Translational Science
Related conditions
bacterial infections, Cancer
Related organisations
IBS Biomedical Mathematics Group, KAIST, POSTECH
Related people
Professor Kim Jae Kyoung (KAIST), Professor Kim Jinsu (POSTECH)