Five recent Parkinson’s disease drug target discoveries
Posted: 10 September 2019 | Victoria Rees (Drug Target Review) | No comments yet
This article highlights five of the latest findings that could be used in the development or design of new therapies to treat Parkinson’s disease.


Scientists investigating Parkinson’s disease (PD) are finding promising therapeutic possibilities that with more research could be used to inform drug development. Here, we list five of the most recent target discoveries for the condition, in order of their journal publication dates, with the newest first.
1. A type of amyloidosis1
Researchers at Osaka University, Japan have discovered that the structure of Parkinson’s disease-associated protein aggregates can provide insight into their movement through the brain. The new findings indicate that the condition is a kind of amyloidosis, which has implications for treatment developments.
It is not yet fully understood why Lewy bodies, primarily composed of α-synuclein proteins, are the neuropathological indicator of PD. The research team used cutting-edge imaging techniques to find that Lewy bodies in Parkinson’s disease brains contain α-synuclein protein aggregates, known as amyloid fibrils, that can propagate through the brain.
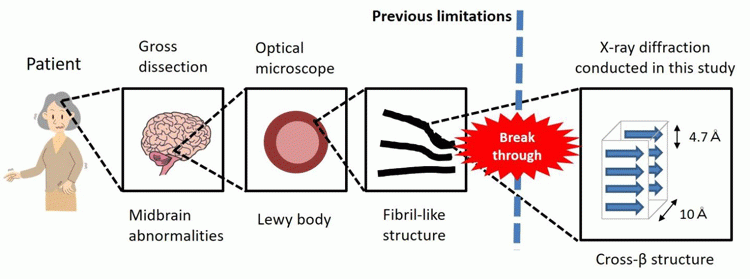

Abnormal changes in the brains of patients with Parkinson’s disease [credit: Osaka University].
“Our work follows on from in vitro findings that aggregates of α-synuclein that can propagate through the brain have a cross-β structure,” says lead author of the study Dr Hideki Mochizuki. “Our study is the first to find that aggregates in Parkinson’s disease brains also have this cross-β structure. This could mean that Parkinson’s disease is a kind of amyloidosis.”
The researchers suggest that PD is a systemic amyloidosis, which is a group of rare diseases caused by abnormal protein accumulation, rather than one that is localised in one part of the brain.
2. Glucose metabolism modification2
An international team of scientists has found a potential drug that may prevent neuronal death through glucose metabolism modification in stressed neurons. The study, using mouse models, has potential therapeutic applications for Parkinson’s disease in humans, according to the researchers.
The researchers were from Gero Discovery LLS, Russia, the Institute of Biomedical Research of Salamanca, Spain and Nanosyn Inc., US.
Glycolysis is generally considered to be the metabolic pathway essential for cell survival, since it meets cell energy needs in case of intensive energy consumption. However, previous studies have shown that in the brain tissue, different cell types show distinct glucose metabolism patterns. In neurons, only a small portion of glucose is consumed via the glycolysis pathway.
…a small molecule, which inhibits PFKFB3, protects neurons from the effects of the condition, as well as other neurological diseases”
At the same time, astrocytes provide nutrients to neurons and utilise glycolysis to metabolise glucose. This is mostly due to the presence of a special protein called PFKFB3, normally absent in neurons and active in astrocytes. In certain neurological diseases like Parkinson’s, the amount of active PFKFB3 increases, which is highly stressful for these cells and leads to cell death.
The research team suggested – and further confirmed with in vivo experiments – that a small molecule, which inhibits PFKFB3, protects neurons from the effects of the condition, as well as other neurological diseases.
“We have already obtained good safety results in mice and believe that we will be successful in our future investigations” said Olga Burmistrova, Director of Pre-clinical Development in Gero Discovery.
The team from Gero is planning to proceed with pre-clinical trials and to move into clinical trials soon.
3. Sex-related differences3

α-synuclein.
According to this piece of research, brain-selective oestrogen treatment improves the symptoms of Parkinson’s disease in male mice. The scientists say that their findings may help to explain the sex-related differences in the condition and could lead to oestrogen-based therapies.
Previous studies have found that oestrogen can protect movement neurons from PD, but its mechanism of action is unknown.
A team from Harvard Medical School, US treated mouse models of PD with brain-selective oestrogen and compared the motor performance of males and females before and after treatment.
They observed that female mice showed less severe symptoms at a later age and oestrogen still improved the condition.
In the male mice, the oestrogen treatment reduced α-synuclein breakdown and build-up. It also helped to relieve severe symptoms, suggesting that oestrogen could be a viable treatment option for Parkinson’s patients.
According to the authors, the “data provide the basis for understanding sex differences in α-synuclein homeostasis and the development of therapeutic approaches to treating men and postmenopausal women,” with Parkinson’s disease.
4. Preventing aggregation4
Research led by the Heinrich Heine University Düsseldorf, Germany, has found a way to prevent α-synuclein from aggregating in the nerve tissue of Parkinson’s patients.
They discovered that a class of engineered binding-proteins, β-wrapins, can block α-synuclein aggregation. This is achieved by capturing the protein monomers and forming chemical complexes with them, which inhibits the elongation of amyloid fibrils.
The β-wrapins also stop seed fibrils from forming in the first place. According to the researchers, only very small amounts of the wrapins are sufficient for this to happen, so a binding protein is not needed for every monomer.
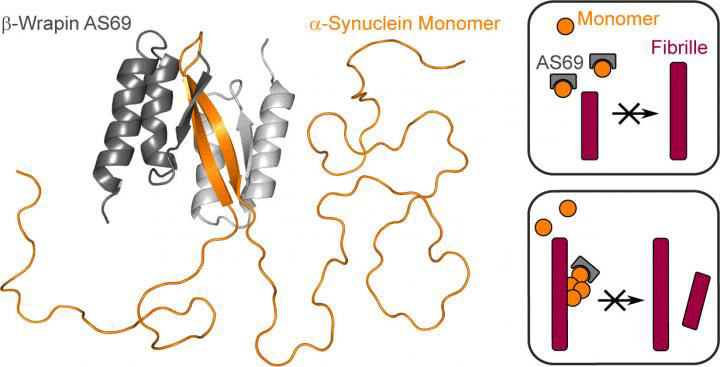

Aggregation inhibitor beta-wrapin AS69 (grey) binds a specific region in the otherwise disordered Parkinson’s protein alpha-synuclein (orange), thus preventing elongation and formation of new protein fibrils (red) [credit: HHU / Wolfgang Hoyer].
“We discovered a few years ago that α-synuclein fibrils can proliferate quickly under certain conditions in a kind of chain reaction. We were really astonished to see that the β-wrapins suppress the chain reactions very efficiently,” said Professor Alexander Büll, Technical University of Denmark. “We think we now understand how this is achieved.”
Using cell cultures and animal models, the researchers were able to treat the β-wrapins, which improved motor skills.
Lead researcher Professor Wolfgang Hoyer said: “The positive results in living creatures give us hope that we have possibly found a path to an active ingredient through the β-wrapins. However, it will still be a long time before this could potentially be used for humans.”
5. DNA repair mechanism5
A study conducted by Oregon Health & Science University, US suggests that Lewy bodies cause problems when they pull α-synuclein out from the nucleus of brain cells. Using mice models and post-mortem brain tissue from humans, the researchers found that the α-synuclein repair DNA breaks.
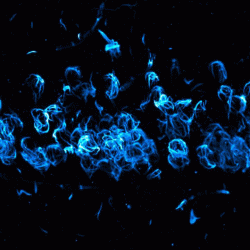

This image shows abnormal aggregated alpha-synuclein Lewy pathology within neurons of brain’s hippocampus in a mouse model of Parkinson’s disease and dementia with Lewy bodies. New research suggests that alpha-nuclein proteins repair DNA breaks within the nucleus of brain cells. When they cluster outside the nucleus, as shown here, it can lead to cellular dysfunction and death [credit: Oregon Health & Science University].
The role of α-synuclein in DNA restoration could therefore be crucial in preventing cell death, but this function may be lost in brain diseases such as Parkinson’s, leading to the widespread death of neurons.
“It may be the loss of that function that’s killing that cell,” said senior author Vivek Unni, MD, PhD, an associate professor of neurology in the OHSU School of Medicine.
The team revealed that the α-synuclein protein is rapidly recruited to the site of DNA damage in the neurons of mice. They also found increased double-strand breaks in the DNA of human tissue and mice in which the protein was clumped together in the form of Lewy bodies in the cytoplasm surrounding the cell’s nucleus.
According to the researchers, the results suggest that α-synuclein plays a crucial role in binding broken strands of DNA within the cell’s nucleus.
The researchers say that their findings could lead to the development of methods to deliver α-synuclein proteins into the nucleus of cells, or design replacements for its function.
References
- Araki K, Yagi N, et al. Parkinson’s disease is a type of amyloidosis featuring accumulation of amyloid fibrils of α-synuclein [Internet]. PNAS. 2019 [cited 6 September 2019]. Available from: https://www.pnas.org/content/116/36/17963
- Burmistrova O, Olias-Arjona A, et al. Targeting PFKFB3 alleviates cerebral ischemia-reperfusion injury in mice [Internet]. Scientific Reports. 2019. [cited 6 September 2019]. Available from: https://www.nature.com/articles/s41598-019-48196-z
- Rajsombath M, Nam A, et al. Female sex and brain-selective estrogen benefit α-synuclein tetramerization and the PD-like motor syndrome in 3K transgenic mice [Internet]. JNeurosci. 2019. [cited 6 September 2019]. Available from: https://www.jneurosci.org/content/early/2019/08/13/JNEUROSCI.0313-19.2019
- Hoyer W, Buell A, et al. An engineered monomer binding-protein for α-synuclein efficiently inhibits the proliferation of amyloid fibrils [Internet]. eLife. 2019 [cited 6 September 2019]. Available from: https://elifesciences.org/articles/46112
- Schaser A, Osterberg V, et al. Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders [Internet]. Scientific Reports. 2019. [cited 6 September 2019]. Available from: https://www.nature.com/articles/s41598-019-47227-z
Related topics
Disease Research, DNA, Drug Development, Drug Targets, Neurons, Neurosciences, Research & Development, Targets
Related conditions
Parkinson's disease (PD)








