Better HIV, lupus and hep C treatments possible with malaria discovery
Posted: 20 November 2019 | Rachael Harper (Drug Target Review) | No comments yet
A new discovery on how the immune system responds to malaria infection could lead to better treatments for hepatitis C, HIV and lupus.
In laboratory models, a research team has showed that strong inflammatory signals caused by malaria infection activate molecules that trigger the production of highly potent antibodies to fight the disease.
The same inflammatory signals are seen in human malaria infections, chronic viral infections and autoimmune disorders. This suggests the discovery could be harnessed to develop new vaccines and therapies that are better able to fight infections such as hepatitis C and HIV as well as treat diseases such as lupus.
The research was led by PhD student Ms Ann Ly and Dr Diana Hansen from the Walter and Eliza Hall Institute, Australia, with collaborators Dr Yiang Liao and Associate Professor Wei Shi and Professor Axel Kallies from the Doherty Institute.
“In our previous paper, we showed that inflammatory signals activated molecules that arrested the development of helper T cells, meaning that B cells did not get the necessary instructions to make antibodies,” Dr Hansen said.
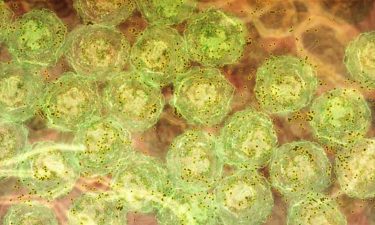
B cells (green) produce antibody (brown). The same inflammatory signals are seen in human malaria infections, chronic viral infections and autoimmunity. This suggests the discovery could be harnessed to develop new vaccines and therapies that are better able to treatments for hepatitis C, HIV and lupus (credit: Walter and Eliza Hall Institute).
“When we began this study, we expected to see that inflammation was also having a negative effect on B cells. In fact, we found the opposite was true. The inflammatory signals were improving the quality of the antibodies produced, by sending B cells to an elite training ground, where they underwent an exhaustive program to become ‘professional predators’.”
Dr Hansen said she hoped that the discovery would have a role beyond malaria, saying that the discovery is “exciting because of the opportunity it offers in treating chronic viral infections and autoimmune disease.
“We have identified the molecular ‘switch’ that drives the immune system to produce highly potent antibodies and the inflammatory signals that influence its function,” she said. “Targeting this molecule, or other molecules in the same pathway, could offer a more ‘precision medicine’ approach to treating these diseases than currently exists.
“The hope is that we would be able to create vaccines or therapies that would ‘switch on’ molecules that help to produce these elite B cells to fight chronic infections better, or ‘switch off’ the same molecules in autoimmune diseases to stop the production of B cells, such as in lupus.”
The research was published in Cell Reports.
Related topics
Antibodies, Disease Research, Drug Development, Drug Targets, Immunology, Research & Development, Targets, Therapeutics
Related conditions
Hepatitis C, HIV, Lupus
Related organisations
Walter and Eliza Hall Institute
Related people
Associate Professor Wei Shi, Dr Diana Hansen, Dr Yiang Liao, Ms Ann Ly, Professor Axel Kallies